World’s Largest Specialty Clinical Data Registry Celebrates its 10-Year Anniversary
Author:
David W. Parke II, MD
As you age, anniversaries tend to sneak up on you. I was reminded that March 24, 2024 marks the 10-year anniversary of the American Academy of Ophthalmology (Academy) IRIS® Registry (Intelligent Research in Sight). I have a special perspective on this milestone, having been part of the ‘obstetrical team’ that helped deliver the IRIS Registry in 2014. Now, 10 years later, I’m still committed to nurturing its growth and success.
An old aphorism holds that ‘success has many fathers, but failure is an orphan.’ By virtually any metric, the IRIS Registry has been a success, and has numerous parents to thank for that. The most notable is Flora Lum, MD, vice president of Quality and Data Science at the Academy—a talented and dedicated colleague. Bill Rich, MD, former Academy medical director for Government Affairs, was one of the very first to really understand the potential of the IRIS Registry and communicate it to the ophthalmologic community. I believe that every Academy board member who voted to fund it and every Academy member ophthalmologist who supported it are part of its parentage.
Less than two years after its launch, over half of ophthalmologists in the United States were actively submitting clinical data to the IRIS Registry.
As I look back, I’m awed by the broad wave of ophthalmic community support that rapidly crested back in 2014. Less than two years after its launch, over half of ophthalmologists in the United States were actively submitting clinical data to the IRIS Registry. No other specialty in medicine had as profound an uptake of its clinical data registry by its members! And it grew from there to become the largest specialty clinical database in all of American medicine.
How did it all happen?
Clinicians and medical organizations have long searched for better tools and better metrics to measure and advance the quality of care delivered to patients. Manual entry registries of patient processes of care and outcomes of that care have been around for generations. They were all cumbersome, costly, slow, and limited in scope. The advent of computer-based clinical records led to some early attempts in the 1990s to aggregate larger datasets from a broader base of patients. This effort was stymied by the slow uptake of electronic health records (EHRs), nonstandardized datasets, and analytic challenges.
The first 10-15 years of the 21st century witnessed the confluence of a number of factors that made the modern clinical data registry possible. EHRs were dramatically improved and adopted by a majority of clinicians, regardless of specialty. Technology permitted the automated extraction of data bits from those EHRs into cloud-based data lakes. Medical information technology groups began defining standards for datasets. And investigators, life sciences companies, clinicians, and health policy experts began to recognize the utility of extremely large datasets in facilitating new science innovation through real world evidence (RWE).
Registries, such as the IRIS Registry, held the promise of using large datasets to better inform the natural history of diseases and response to treatments, to understand true patterns of clinical practice in the ‘real world,’ to compare the effectiveness of various diagnostic and therapeutic options, and to study rare diseases with unheard of granularity and statistical significance. And for clinicians, they held the promise of allowing individuals to better understand their own practices, their patterns of care, their patients’ outcomes, to benchmark these against peers and norms, and to measure the impact of improvements.

How does it work?
The broad architecture is straightforward. EHR data are stripped of all unique patient identifiers for patient privacy purposes and are uploaded into secure cloud-based servers of the IRIS Registry and then analyzed digitally. The Academy owns the IRIS Registry and the de-identified data.
The complex technologies and cost explain, quite frankly, why a number of registries have failed—and why Verana Health’s capabilities are so critical to the IRIS Registry and to the Academy.
The actual process of ingestion of data into the IRIS Registry, and the curation and analysis of data to yield useful results, are much more complex. The complex technologies and cost explain, quite frankly, why a number of registries have failed—and why Verana Health’s capabilities are so critical to the IRIS Registry and to the Academy. Consider the following examples:
- EHRs – Ophthalmologists use over 60 different EHRs. Not only does, for example, ‘right eye intraocular pressure’ exist in a field within a different location in each of these EHRs, but the customization performed for individual practices means that there are really close to 1,000 ‘different EHRs.’ The location of a desired piece of data in each EHR must be mapped to the IRIS Registry field architecture. And each time the EHR software is updated, the mapping may require adjustment. In aggregate, this occurs thousands of times a year!
- Data format – At one point, in examining the way a large ophthalmology practice recorded visual acuity, data scientists at Verana Health encountered over 50 different forms. For example, a Snellen visual acuity of missing two letters on the 20/40 line may be recorded as ‘20/40,’ ‘20/40-2,’ ‘20/40 minus 2,’ ‘20/40 minus two,’ ‘20/50 plus,’ ‘20/40 minus,’ ‘almost 20/40,’ or an entirely different ETDRS format.
- Unstructured data – Some data are structured and numerical (e.g., visual acuity), some are structured but not numerical (e.g., a specific field to indicate “yes/no”), but much is unstructured free text in EHR clinical notes. Curation of free text has been transformed by artificial intelligence (AI) algorithms but remains complex. Key terms are generally unstandardized and may not predictably always be resident in specific fields.
Accurate curation and analysis requires the input of engineers, data scientists, physicians, epidemiologists, and experienced clinical trialists. It becomes even more complex with linkage to non-IRIS Registry data from areas such as imaging, genomics, and clinical claims.
What has been the impact?
Over 100 peer-reviewed scientific articles have been published in the peer-reviewed literature using data derived from the IRIS Registry. This has added tremendously to the science of ophthalmology and vision. Over 250 invited papers have been presented. Verana Health has worked collaboratively with the U.S. Food and Drug Administration (FDA) and with the National Eye Institute of the National Institutes of Health (NIH) on issues as diverse as clinical imaging, pediatric cataract surgery, and development of patient-reported outcomes tools. With more than 605 million billable visits from more than 80 million unique de-identified patients in the IRIS Registry database, the power of ‘big data’ becomes evident.
One of the biggest outcomes has been in the area of quality of care measures. Under the federal Merit-based Incentive Payment System (MIPS), ophthalmologists can utilize Verana Health to submit IRIS Registry data as evidence of compliance with federal quality measures to avoid payment penalties under Medicare. These data are submitted using the Verana Quality Measures Dashboard. Since 2017, over 10,000 IRIS Registry participating ophthalmologists have avoided more than $1.4 billion in federal penalties using the IRIS Registry and Verana Health’s advanced technology. This amounts to an average of over $36,000 per ophthalmologist, per year.
IRIS Registry data are also embedded in the Verana Trial Connect tool that provides participating clinicians with a curated list of their own practice patients that have a high potential for clinical trials recruitment relative to specific inclusion and exclusion criteria. The Verana Research Network is a growing clinical trials network of active trial locations. Together, these two services have the potential to help accelerate the faster and more efficient identification of a larger group of potentially study-eligible patients for important and time-sensitive clinical trials.
What is Verana Health’s role and how critical is it to the IRIS Registry?
The Academy owns the IRIS Registry and solely funded its initial development. It was anticipated and became rapidly apparent that sustainability would require monetization of the IRIS Registry data asset (in a way consistent with appropriate privacy and data security concerns and with the policies of the Academy). It also became apparent that this would require a public-private partnership between the Academy and a for-profit corporate partner. That data curation and analytics partner is Verana Health, Inc., which is governed by a board of directors representing investors, physicians, and leaders of medical organizations. Verana Health is the exclusive data analytics partner for registries in ophthalmology, neurology, and urology. In return for licensing commercialization of data under agreements with the medical societies, Verana Health covers costs associated with data ingestion and storage, and with curation and data analytics—a value of millions of dollars annually to each of the societies. Without the support of Verana Health, the registries would likely not exist in their current form or be the robust contributors to medical science that they have become.
Verana Health collaborates with life sciences companies to provide actionable information pertaining to disease natural history, treatment comparative effectiveness, clinical trials support, and other uses of de-identified clinical data that can help companies provide therapeutic innovations to patients and their physicians.
What may the future hold for the IRIS Registry?
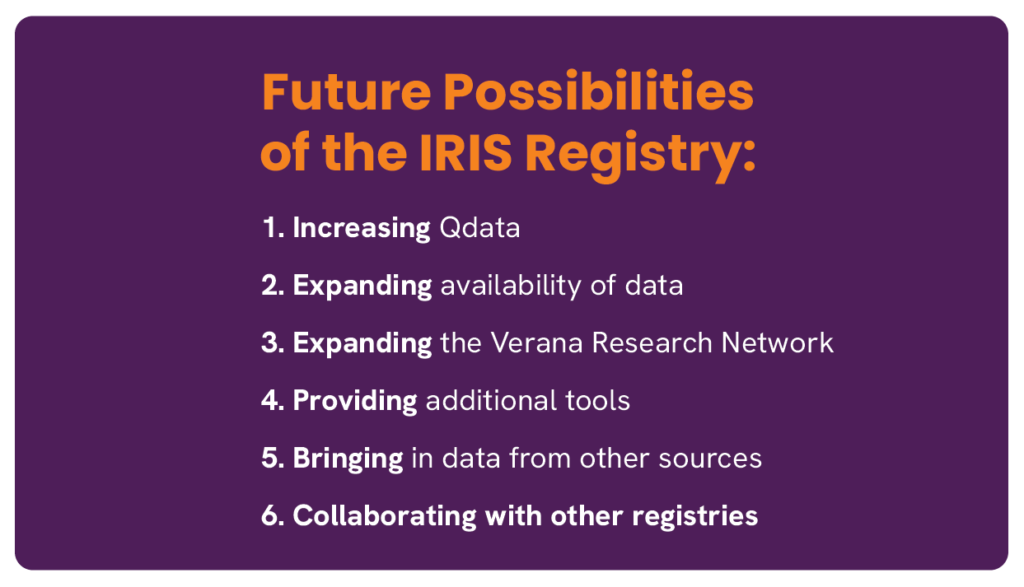
I firmly believe that we have yet to realize anything close to the full potential of the IRIS Registry to help ophthalmologists advance the care they can deliver, and life sciences companies to optimally plan for the future. Options on the table include:
- Increasing the number of structured quality data (Qdata) sets to include more and more diseases (Verana Health currently has 8 in ophthalmology). Qdata are curated datasets ready for use in RWE-based research projects.
- Expanding availability to vision scientists and clinical ophthalmologists of the IRIS Registry data to advance the science of our shared profession.
- Expanding the Verana Research Network to assist both ophthalmologists and life sciences companies with focused and more rapid development of clinical trials involvement.
- Providing tools for individual ophthalmic practices in areas of surgical and treatment outcomes measurement and benchmarking, and in the area of practice management.
- Bringing into the IRIS Registry data from other sources that will further enrich the database used for research and patient care.
- Collaborating with other registries to facilitate better data standards. One outcome of this initiative should be the very important capability to include data fields from other registries so that (for example) study of retinal vascular disease might advantage itself of data in a cardiovascular disease registry.
Both the Academy and Verana Health are benefitting tremendously from this special IRIS Registry partnership. The Academy is relieved of many of the significant engineering and human costs associated with a clinical data registry and can sustain a program of great value to its members and the profession. Verana Health has an asset that is central to propelling its commercial effectiveness and growth. And it can tap into the substantial professional expertise of the Academy.
Above all, patients and clinicians benefit. Incentives are aligned in wanting to advance the availability of the drugs and tools we have as ophthalmologists to render the best care to as many patients as possible.
It has been my great pleasure, first as CEO of the Academy and now as executive chair of Verana Health, to help bring this dream closer to fruition.

Let's Accelerate Research Together
To learn more about Verana Health, please fill out the information below and our team will follow up with you as soon as possible.

